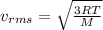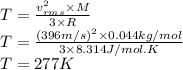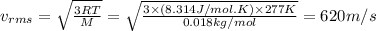Chemistry, 13.03.2020 05:04 elianagilbert3p3hh63
The root-mean-square speed (thermal speed) of a certain sample of carbon dioxide molecules, with a molecular weight of 44 g/mol, is 396 m/s. What is the root-mean-square speed (thermal speed) of water vapor molecules, with a molecular weight of 18 g/mol, at the same temperature?

Answers: 1


Another question on Chemistry

Chemistry, 23.06.2019 00:10
Covalent compounds: mastery test select the correct answer what is formed when atoms join together with a covalent bond? a. an ion b. a molecule c. a neutral atom d. a noble gas
Answers: 3

Chemistry, 23.06.2019 00:30
Which radioisotope is used to date fossils? a. oxygen-16 b. carbon-14 c. uranium-238 d. carbon-12
Answers: 2

Chemistry, 23.06.2019 03:00
Describe the properties of sodium, chlorine, and sodium chloride
Answers: 1

Chemistry, 23.06.2019 07:00
Why do the strengths of london (dispersion) forces generally increase with increasing molecular size? choose one: a. heavier atoms have stronger attractions for each other than lighter atoms. b. dispersion forces are all equal in magnitude; there is no size dependence. c. dispersion forces arise from the attraction between the nuclei of atoms, and larger molecules have larger nuclei. d. dispersion forces arise from dipoles caused by the electron distribution being distorted. larger molecules have more electrons and, therefore, more distortions and a bigger force. e. dispersion forces depend on distance. larger molecules are farther apart and so the forces are smaller.
Answers: 2
You know the right answer?
The root-mean-square speed (thermal speed) of a certain sample of carbon dioxide molecules, with a m...
Questions

Mathematics, 18.06.2021 01:10

Chemistry, 18.06.2021 01:10





Computers and Technology, 18.06.2021 01:10











Mathematics, 18.06.2021 01:10

Mathematics, 18.06.2021 01:10

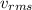 ) of a gas can be calculated using the following expression.
) of a gas can be calculated using the following expression.