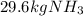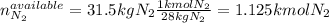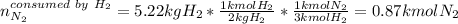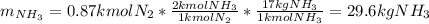Chemistry, 13.03.2020 02:27 ddddre3909
Ammonia can also be synthesized by the reaction: 3H2(g)+N2(g)→2NH3(g) What is the theoretical yield of ammonia, in kg, that we can synthesize from 5.22 kg of H2 and 31.5 kg of N2? Express the mass in kilograms to three significant figures.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
Needthe meter is the standard unit for: 1) height 2) length 3) weight 4) mass
Answers: 3

Chemistry, 22.06.2019 04:00
Write the empirical chemical formula of calcium with a mass percent of 38.8, phosphorus with a mass percent of 20.0, and oxygen with a mass percent of 41.3.
Answers: 1

Chemistry, 22.06.2019 07:30
What is i fracture in the crust called when land move up, down or sideways
Answers: 2

Chemistry, 22.06.2019 12:10
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3
You know the right answer?
Ammonia can also be synthesized by the reaction: 3H2(g)+N2(g)→2NH3(g) What is the theoretical yield...
Questions

Mathematics, 07.07.2019 04:20



History, 07.07.2019 04:20

Mathematics, 07.07.2019 04:20

Mathematics, 07.07.2019 04:20

History, 07.07.2019 04:20

Chemistry, 07.07.2019 04:20

History, 07.07.2019 04:20

History, 07.07.2019 04:20



Mathematics, 07.07.2019 04:20


English, 07.07.2019 04:20


Social Studies, 07.07.2019 04:20



Biology, 07.07.2019 04:20