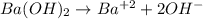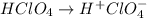Chemistry, 13.03.2020 02:26 valeriegarcia12
An aqueous solution of barium hydroxide is standardized by titration with a 0.167 M solution of perchloric acid. If 12.8 mL of base are required to neutralize 25.4 mL of the acid, what is the molarity of the barium hydroxide solution?

Answers: 2


Another question on Chemistry




Chemistry, 23.06.2019 04:10
What does the field of thermodynamics relate to a-changes in nuclear reactions b- changes in energy in systems c changes in molecular structure d changes in atomic properties
Answers: 1
You know the right answer?
An aqueous solution of barium hydroxide is standardized by titration with a 0.167 M solution of perc...
Questions

Mathematics, 01.09.2021 15:00

Mathematics, 01.09.2021 15:00

World Languages, 01.09.2021 15:00


Mathematics, 01.09.2021 15:00


Geography, 01.09.2021 15:00

Mathematics, 01.09.2021 15:00


Biology, 01.09.2021 15:10

Mathematics, 01.09.2021 15:10

Social Studies, 01.09.2021 15:10

Mathematics, 01.09.2021 15:10


English, 01.09.2021 15:10

Mathematics, 01.09.2021 15:10

English, 01.09.2021 15:10

History, 01.09.2021 15:10