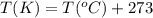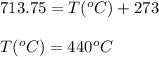Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 11:30
Compare and contrast refraction of light and sound will give brainliest
Answers: 1

Chemistry, 22.06.2019 19:00
How many liters of ethylene glycol antifreeze (c2h6o2), with a density of 1.100 g/l, would you add to your car radiator containing 15.0 kg of water if you needed to protect your engine to - 21.5°c? for water, kf = 1.86°c m -1.
Answers: 1


Chemistry, 23.06.2019 14:00
Cassandra made a venn diagram to compare and contrast the two stages of cellular respiration. which belongs in the area marked x? energy is released. oxygen is used up. glucose is broken down. carbon dioxide is used up.
Answers: 1
You know the right answer?
A sample of gas has a volume of 1.24 L under 2.35 atm pressure at 45°C. If the gas is then expanded...
Questions






Mathematics, 07.03.2020 04:44





Mathematics, 07.03.2020 04:44



Mathematics, 07.03.2020 04:44






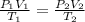
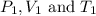 are the initial pressure, volume and temperature of the gas
are the initial pressure, volume and temperature of the gas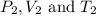 are the final pressure, volume and temperature of the gasW
are the final pressure, volume and temperature of the gasW![P_1=2.35atm\\V_1=1.24L\\T_1=45^oC=[45+273]K=318K\\P_2=0.515atm\\V_2=12.7L\\T_2=?](/tpl/images/0544/2731/0dccc.png)