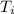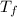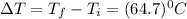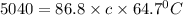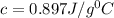Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 22.06.2019 23:00
Which organism develops breathing organism develops breathing organs from pharyngeal arches? shark, spider, sea star, sea horse
Answers: 2

Chemistry, 22.06.2019 23:30
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
An unknown metal has a mass of 86.8 g. When 5040 J of heat are added to the sample, the sample tempe...
Questions

Mathematics, 20.01.2021 08:30


Health, 20.01.2021 08:30



Mathematics, 20.01.2021 08:40

Mathematics, 20.01.2021 08:40

Biology, 20.01.2021 08:40




Mathematics, 20.01.2021 08:40



Mathematics, 20.01.2021 08:40

Chemistry, 20.01.2021 08:40

Mathematics, 20.01.2021 08:40

World Languages, 20.01.2021 08:40

Mathematics, 20.01.2021 08:40

Computers and Technology, 20.01.2021 08:40

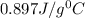
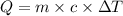
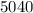 Joules
Joules