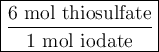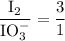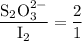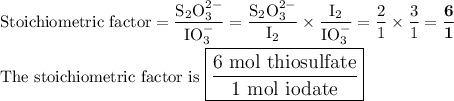Part III. The two reactions involved in quantitatively determining the amount of iodate in solution are: IO3-(aq) 5 I-(aq) 6 H (aq) --> 3 I2(aq) 3 H2O(l) followed by reaction of the I2: I2(aq) 2 S2O32- --> 2 I-(aq) S4O62-(aq). What is the stoichiometric factor, that is the number of moles of Na2S2O3 reacting with one mole of KIO3

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 07:20
Which of these conditions most likely produces an unstable isotope?
Answers: 1

Chemistry, 22.06.2019 09:00
The diagram below shows a cell placed in a solution.a cell is shown placed inside a beaker. it is labeled cell. the solution inside the beaker is labeled 40% salt solution and the solution inside the cell is labeled 20% salt solution.only water is allowed to move in and out of the cell. what will most likely happen to the cell? it will expand as water moves out of it. it will shrink as water moves out of it.it will expand as water moves into it. it will shrink as water moves into it.
Answers: 2

Chemistry, 22.06.2019 18:10
The atom fluorine generally will become stable through the formation of an ionic chemical compound by accepting electron(s) from another atom. this process will fill its outer energy level of electrons.
Answers: 1
You know the right answer?
Part III. The two reactions involved in quantitatively determining the amount of iodate in solution...
Questions


Physics, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01

Mathematics, 12.08.2020 06:01


Mathematics, 12.08.2020 06:01


Mathematics, 12.08.2020 06:01








Mathematics, 12.08.2020 06:01


Health, 12.08.2020 06:01