
At a certain temperature the rate of this reaction is first order in HI with a rate constant of :7.21s?1
2HI(g)??H2(g) + I2(g)
Suppose a vessel contains HI at a concentration of 0.440M
. Calculate the concentration of HI in the vessel 0.210 seconds later. You may assume no other reaction is important.
Round your answer to 2 significant digits.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
The human activities in two locations are described below: location a: rampant use of plastic containers location b: excessive use of pesticides and fertilizers which statement is most likely true? location a will have poor air quality because plastic is biodegradable. location a will experience water scarcity because plastic absorbs moisture. the population of honeybees will increase in location b because production of crops will increase. the population of fish in location b will decrease because the water is contaminated.
Answers: 1

Chemistry, 22.06.2019 19:20
15. which of the following is not human-caused groundwater pollution? a. water in an aquifer dissolves elements such as arsenic and mercury from surrounding rock. b. water in an aquifer is contaminated by leachate that seeps into the ground from a landfill. c. water in an aquifer becomes polluted with chemicals used in hydraulic fracturing, or fracking. d. water in an aquifer absorbs harmful bacteria from the drainage field of a septic tank.
Answers: 1

Chemistry, 22.06.2019 19:30
To calculate percent by mass, use the equation below: calculate the percent by mass of each element. %n = % %h = % %o = %
Answers: 3

Chemistry, 22.06.2019 23:00
If two identical atoms are bonded,what kind of molecule is formed
Answers: 1
You know the right answer?
At a certain temperature the rate of this reaction is first order in HI with a rate constant of :7.2...
Questions

Health, 15.12.2020 17:40


Mathematics, 15.12.2020 17:40

Mathematics, 15.12.2020 17:40

Mathematics, 15.12.2020 17:40

Mathematics, 15.12.2020 17:40

English, 15.12.2020 17:40


Mathematics, 15.12.2020 17:40


Biology, 15.12.2020 17:40

English, 15.12.2020 17:40

Chemistry, 15.12.2020 17:40

Chemistry, 15.12.2020 17:40

Computers and Technology, 15.12.2020 17:40

Mathematics, 15.12.2020 17:40


Mathematics, 15.12.2020 17:40

History, 15.12.2020 17:40

Social Studies, 15.12.2020 17:40

![[A_o]=0.440 M](/tpl/images/0541/4407/bdc04.png)
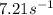
![[A]=[A_o]\times e^{-kt}](/tpl/images/0541/4407/abdec.png)
![[A]=0.440 M\times e^{-7.21 s^{-1}\times 0.210 s}](/tpl/images/0541/4407/4243c.png)


