

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:00
Drag each label to the correct location on the chart. classify each reaction as endothermic or exothermic.
Answers: 1

Chemistry, 22.06.2019 13:30
Which is true of a liquid? it has a definite volume but not a definite mass.it has a definite mass but not a definite volume.it has a definite volume but not a definite shape.it has a definite shape but not a definite volume.
Answers: 2

Chemistry, 22.06.2019 16:00
Click the button that shows the correct relationship of the electron affinities of the elements sodium and phosphorus. sodium’s electron affinity value is more negative than the electron affinity value of phosphorus. phosphorus’ electron affinity value is more negative than the electron affinity value of sodium. this information cannot be determined using the periodic table. answer is b on e2020.
Answers: 3

Chemistry, 22.06.2019 22:30
Which of the following is true about the speed of light? it depends on the wavelength.
Answers: 3
You know the right answer?
Calculate the mass of H2O produced by metabolism of 1.2 kg of fat, assuming the fat consists entirel...
Questions

Social Studies, 06.06.2020 05:58

Biology, 06.06.2020 05:58

English, 06.06.2020 05:58




Mathematics, 06.06.2020 05:58

Mathematics, 06.06.2020 05:58


Biology, 06.06.2020 05:58

Law, 06.06.2020 05:58

Mathematics, 06.06.2020 05:58


Mathematics, 06.06.2020 05:58

Biology, 06.06.2020 05:58

Mathematics, 06.06.2020 05:58

History, 06.06.2020 05:58

Advanced Placement (AP), 06.06.2020 05:58

Mathematics, 06.06.2020 05:58

Mathematics, 06.06.2020 05:58

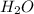 is produced
is produced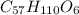 +
+ 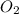 ⇒
⇒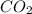 +
+ 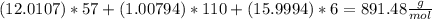
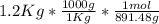
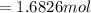
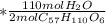
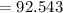 mol
mol 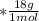 =1665.7 g
=1665.7 g 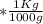 ≅ 1.7 Kg
≅ 1.7 Kg

