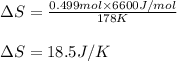Chemistry, 10.03.2020 07:25 hhvgbv49551
The heat of fusion ΔHf of toluene C6H5CH3 is 6.6 /kJmol . Calculate the change in entropy ΔS when 46.g of toluene melts at −95.0°C

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:10
According to the diagram; a) identify the anode of the cell and write the half-reaction that occurs there b) write the overall equation for the reaction that occurs as the cell operates c) calculate the value of the standard cell potential ,e cell. d)write the shorthand notation of the cell above e)indicate the flow of the electrons on the diagram
Answers: 3

Chemistry, 23.06.2019 00:00
How many atoms or molecules are there in a mole of a substance?
Answers: 1

Chemistry, 23.06.2019 03:00
Can someone me out on this question for my national 5 chemistry homework
Answers: 1

Chemistry, 23.06.2019 10:10
Solid tin exists in two forms: white and gray. for the transformation sn(s, white) → sn(s, gray) the enthalpy change is -2.1 kj/mol and the entropy change is -7.4 j/(mol*k). a. calculate the gibbs free energy change for the conversion of 1.00 mol white tin to gray tin at -30℃. b. will white tin convert spontaneously to gray tin at -30℃? c. at what temperature are white and gray tin thermodynamically equivalent at a pressure of 1 atm?
Answers: 3
You know the right answer?
The heat of fusion ΔHf of toluene C6H5CH3 is 6.6 /kJmol . Calculate the change in entropy ΔS when 46...
Questions

Mathematics, 22.09.2021 21:20

Spanish, 22.09.2021 21:20

Mathematics, 22.09.2021 21:20

Mathematics, 22.09.2021 21:20


Mathematics, 22.09.2021 21:20

English, 22.09.2021 21:20

Mathematics, 22.09.2021 21:20

Mathematics, 22.09.2021 21:20


Mathematics, 22.09.2021 21:20

Social Studies, 22.09.2021 21:20

History, 22.09.2021 21:20

Physics, 22.09.2021 21:20






Mathematics, 22.09.2021 21:20

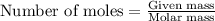
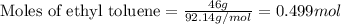
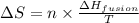
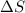 = Entropy change = ?
= Entropy change = ?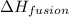 = enthalpy of fusion = 6.6 kJ/mol = 6600 J/mol (Conversion factor: 1 kJ = 1000 J)
= enthalpy of fusion = 6.6 kJ/mol = 6600 J/mol (Conversion factor: 1 kJ = 1000 J)![-95.0^oC=[-95.0+273]K=178K](/tpl/images/0540/3339/0d1c2.png)