
Chemistry, 10.03.2020 00:16 SandyRiverty
Balance the chemical equation by adding coefficients as needed. equation: (a) FeCl2(aq) + KMnO4(aq) + HCl(aq) -> FeCl3(aq) + MnCl2(aq) + H2O(l) + KCl(aq) (b) FeCl2 (aq) + KMnO4(aq) + HCl (aq) ⟶ FeCl3 (aq) + MnCl(aq) + H2O(l) + KCl (aq)

Answers: 3


Another question on Chemistry


Chemistry, 21.06.2019 21:30
It takes 945.kj/mol to break a nitrogen-nitrogen triple bond. calculate the maximum wavelength of light for which a nitrogen-nitrogen triple bond could be broken by absorbing a single photon.
Answers: 3

Chemistry, 21.06.2019 22:30
Each of the following compounds contains a metal that can exhibit more than one ionic charge. provide systematic names for each of these compounds. (a) cr(clo3)6 (b) mo(cn)6 (c) cr2(so3)3 (d) v(clo2)2 (e) v(cn)5 (f) os(clo2)4
Answers: 3

Chemistry, 22.06.2019 02:10
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3
You know the right answer?
Balance the chemical equation by adding coefficients as needed. equation: (a) FeCl2(aq) + KMnO4(aq)...
Questions












English, 13.03.2020 01:48


Mathematics, 13.03.2020 01:48

History, 13.03.2020 01:48



Mathematics, 13.03.2020 01:49


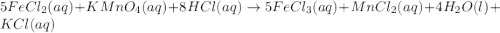
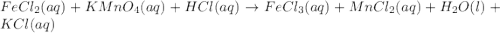
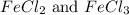 , the coefficient '8' put before the
, the coefficient '8' put before the 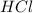 and the coefficient '4' put before the
and the coefficient '4' put before the 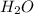 and we get the balanced chemical equation.
and we get the balanced chemical equation.

