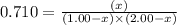Consider the reaction, which takes place at a certain elevated temperature CO(g)+NH3(g)⇌HCONH2(g), Kc=0.710 If a reaction vessel initially contains only CO and NH3 at concentrations of 1.00 M and 2.00 M, respectively, what will the concentration of HCONH2 be at equilibrium?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 18:00
Aballoon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon? a balloon inflated with three breaths of air has a volume of 1.7 l. at the same temperature and pressure, what is the volume of the balloon if five more same-sized breaths are added to the balloon?
Answers: 3

Chemistry, 22.06.2019 00:00
Which of the following methods uses the decay of atomic particles in an object to find its exact age? a. fossil dating b. geologic dating c. radioactive dating d. relative dating
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 18:00
How many moles of oxygen gas are produced from the decomposition of six moles of potassium chlorate
Answers: 3
You know the right answer?
Consider the reaction, which takes place at a certain elevated temperature CO(g)+NH3(g)⇌HCONH2(g), K...
Questions





Mathematics, 31.07.2019 23:00



Mathematics, 31.07.2019 23:00




Mathematics, 31.07.2019 23:00

Mathematics, 31.07.2019 23:00


History, 31.07.2019 23:00

Mathematics, 31.07.2019 23:00

Social Studies, 31.07.2019 23:00



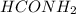 at equilibrium is, 0.513 M
at equilibrium is, 0.513 M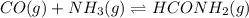
![K_c=\frac{[HCONH_2]}{[CO][NH_3]}](/tpl/images/0539/2269/5249e.png)