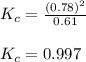Chemistry, 09.03.2020 23:59 pollywallythecat
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and is allowed to reach equilibrium described by the equation N2O4(g) 2NO2(g) If at equilibrium the N2O4 is 39% dissociated, what is the value of the equilibrium constant, Kc, for the reaction under these conditions

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 21.06.2019 22:30
Often on a topographic map, every fifth contour line is darkened. what is this line called? a. key b.slope c.benchmark d. index contour
Answers: 1

Chemistry, 22.06.2019 05:30
Why is soap used to remove grease? a. its nonpolar end dissolves the grease. b. it makes the water bond with the grease. c. it chemically bonds with the grease. d. its polar end dissolves the grease.correct answer for apex - a, its nonpolar end dissolves the grease.
Answers: 1

Chemistry, 22.06.2019 09:30
Melissa is interested in her family tree and how her family has changed over its many generations. melissa probably more closely resembles
Answers: 2
You know the right answer?
Exactly 1.0 mol N2O4 is placed in an empty 1.0-L container and is allowed to reach equilibrium descr...
Questions

Mathematics, 17.12.2020 22:20

English, 17.12.2020 22:20

Mathematics, 17.12.2020 22:20

Geography, 17.12.2020 22:20

Mathematics, 17.12.2020 22:20

Mathematics, 17.12.2020 22:20




Mathematics, 17.12.2020 22:20



Biology, 17.12.2020 22:20




Mathematics, 17.12.2020 22:20



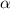 = 0.39
= 0.39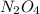 , c =
, c = 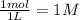
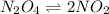
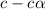
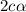
![N_2O_4=c-c\alpha =[1-(1\times 0.39)]=0.61M](/tpl/images/0539/1468/f8a46.png)
![NO_2=2c\alpha =[2\times 1\times 0.39]=0.78M](/tpl/images/0539/1468/5fcc4.png)
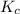 for above equation follows:
for above equation follows:![K_{c}=\frac{[NO_2]^2}{[N_2O_4]}](/tpl/images/0539/1468/1a559.png)