
Chemistry, 09.03.2020 17:02 Kalle91106
Quantum numbers arise naturally from the mathematics used to describe the possible states of an electron in an atom.
The four quantum numbers, the principal quantum number (n), the angular momentum quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms) have strict rules which govern the possible values.
Identity allowable combinations of quantum numbers for an electron. Select all that apply.
a) n = 3, l = 2, ml = −2, ms = −1/2
b) n = 4, l = 0, ml = 1, ms = −1/2
c) n = 3, l = −2, ml = −2, ms = +1/2
d) n = 5, l = 4, ml = 4, ms = +1/2
e) n = 3, l = 3, ml = 1, ms = +1/2
f) n = 2, l = 1, ml = 0 ,ms = 0

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:30
Identify the missing numbers below to show the result of multiplying the numbers (1.6 × 10-19)(5.0 × 106) = c × 10d
Answers: 1

Chemistry, 22.06.2019 12:30
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Chemistry, 23.06.2019 03:00
Analyze the reaction to determine whether the reaction is exothermic or endothermic. explain your reasoning.
Answers: 1

Chemistry, 23.06.2019 03:30
Mr. rose asked his student to draw a quadrilateral with four unequal sides. an example of this kind of quadrilateral
Answers: 1
You know the right answer?
Quantum numbers arise naturally from the mathematics used to describe the possible states of an elec...
Questions


Social Studies, 05.03.2020 08:44






History, 05.03.2020 08:45












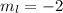 ,
, 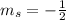
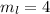 ,
, 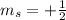
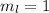 ,
, 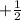 for upward spin and
for upward spin and 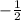 for downward spin.
for downward spin.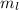 can have values from -l to +l i.e for l= 2 ,
can have values from -l to +l i.e for l= 2 , 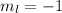 ,
, 

