
Chemistry, 07.03.2020 05:26 juanesmania
A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressure conditions. It required 105 s for 1.0 L of the gas to effuse. Under identical experimental conditions it required 30 s for 1.0 L of O2 gas to effuse. Calculate the molar mass of the unknown gas.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Which of the following two events occur to create a sea breeze? select all that apply. warm air rises on the ocean and moves toward the land to cool warm air rises on land and moves toward the ocean to cool cool air moves from the ocean to be warmed by the land cool air moves from the land to be warmed by the ocean
Answers: 3


Chemistry, 22.06.2019 15:00
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 18:30
Which rate indicates the number of children that would be born per woman if she were to live to the end of her child bearing years
Answers: 2
You know the right answer?
A gas of unknown molecular mass was allowed to effuse through a small opening under constant-pressur...
Questions

Social Studies, 02.09.2019 04:30

Arts, 02.09.2019 04:30

Mathematics, 02.09.2019 04:30


Biology, 02.09.2019 04:30

Chemistry, 02.09.2019 04:30

Biology, 02.09.2019 04:30


History, 02.09.2019 04:30

Mathematics, 02.09.2019 04:30

Mathematics, 02.09.2019 04:30

History, 02.09.2019 04:30

Computers and Technology, 02.09.2019 04:30


Mathematics, 02.09.2019 04:30

Mathematics, 02.09.2019 04:30

English, 02.09.2019 04:30

Mathematics, 02.09.2019 04:30

History, 02.09.2019 04:30

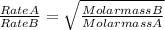
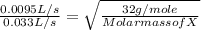 or Molar mass of X =
or Molar mass of X = 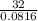 g/mole = 391.99 g/mole
g/mole = 391.99 g/mole

