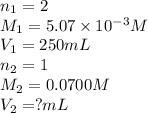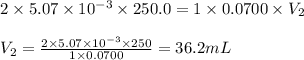A chemistry student weighs out of sulfurous acid , a diprotic acid, into a volumetric flask and dilutes to the mark with distilled water. He plans to titrate the acid with solution. Calculate the volume of solution the student will need to add to reach the final equivalence point. Be sure your answer has the correct number of significant digits.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3

Chemistry, 22.06.2019 07:30
Compare and contrast the bohr model and the electron cloud models of the atom.
Answers: 1

Chemistry, 22.06.2019 09:00
An excess of lithium oxide undergoes a synthesis reaction with water to produce lithium hydroxide li2o+h2o→2lioh if 1.05 g of water reacted, what is the theoretical yield of lithium hydroxide? a) 5.83 x 10–2 g lioh b) 1.17 x 10–1 g lioh c) 2.79 x 100 g lioh d) 1.40 x 100 g lioh
Answers: 1

You know the right answer?
A chemistry student weighs out of sulfurous acid , a diprotic acid, into a volumetric flask and dilu...
Questions


Mathematics, 12.02.2021 16:30


Mathematics, 12.02.2021 16:30

Mathematics, 12.02.2021 16:30

Mathematics, 12.02.2021 16:30

Biology, 12.02.2021 16:30





History, 12.02.2021 16:30



Physics, 12.02.2021 16:30

Mathematics, 12.02.2021 16:30

Mathematics, 12.02.2021 16:30

Mathematics, 12.02.2021 16:30

Mathematics, 12.02.2021 16:30

Mathematics, 12.02.2021 16:30

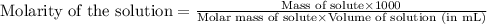
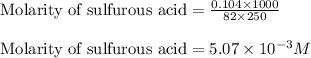
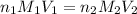
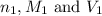 are the n-factor, molarity and volume of acid which is
are the n-factor, molarity and volume of acid which is 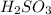
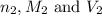 are the n-factor, molarity and volume of base which is NaOH.
are the n-factor, molarity and volume of base which is NaOH.