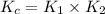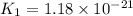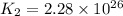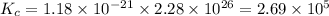Consider the following reactions and their equilibrium constants at 303 K. PCl5(g) equilibrium reaction arrow 1/4 P4(g) + 5/2 Cl2(g); Kc = 1.18 ✕ 10−21 1/4 P4(g) + 3/2 Cl2(g) equilibrium reaction arrow PCl3(g); Kc = 2.28 ✕ 1026 Calculate Kc for PCl5(g) equilibrium reaction arrow PCl3(g) + Cl2(g) at the same temperature.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2

Chemistry, 22.06.2019 19:00
Which is the solubility product expression for caf2(s)?  [ca2+]/[f–]2  [ca2+][f2–]  [ca]+[f]2  [ca2+][f–]2
Answers: 3

Chemistry, 22.06.2019 20:00
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
Consider the following reactions and their equilibrium constants at 303 K. PCl5(g) equilibrium react...
Questions


Chemistry, 13.04.2020 19:00



Mathematics, 13.04.2020 19:00

English, 13.04.2020 19:00



Health, 13.04.2020 19:00

Biology, 13.04.2020 19:00


Spanish, 13.04.2020 19:00



Mathematics, 13.04.2020 19:00


Mathematics, 13.04.2020 19:00

Mathematics, 13.04.2020 19:00


Mathematics, 13.04.2020 19:00

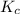 for the net reaction is
for the net reaction is 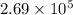
![PCl_5(g)\xrightarrow[]{K_1} \frac{1}{4}P_4(g)+\frac{5}{2}Cl_2(g)](/tpl/images/0537/3407/f38c5.png)
![\frac{1}{4}P_4(g)+\frac{3}{2}Cl_2(g)\xrightarrow[]{K_2} PCl_5(g)](/tpl/images/0537/3407/948a1.png)
![PCl_5(g)\xrightarrow[]{K_c} PCl_3(g)+Cl_2(g)](/tpl/images/0537/3407/3c32f.png)