

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Will mark brainliest 26. which of these statements are true? (3 points) a. gases are compressible b. gases fill their containers completely c. the pressure of a gas is independent of the temperature d. gases have mass e. gases exert pressure f. the pressure of a gas is dependent on the volume g. gas pressure results from the collisions between gas particles h. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 06:30
Three cards with holes are arranged in a straight line. a light is shined through the first card’s hole and travels through all three cards. what does this tell you about light rays? a) that light is reflected b) that light is refractive c) that light travels in a straight line d) that light does not travel in a straight line
Answers: 1

Chemistry, 22.06.2019 07:50
In which situation can a mixture always be called a solution
Answers: 3

Chemistry, 22.06.2019 21:50
If e is the symbol for an element, which two of the following symbols represent isotopes of the same element? 1. e2. e3. ea.1 and 2c.1 and 4b.3 and 4d.2 and 3
Answers: 2
You know the right answer?
Determine the theoretical yield of H2S (in moles) if 4.0 molAl2S3 and 4.0 mol H2O are reacted accord...
Questions


Mathematics, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30

History, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30

Computers and Technology, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30


Mathematics, 18.03.2021 03:30

Chemistry, 18.03.2021 03:30

Arts, 18.03.2021 03:30

Mathematics, 18.03.2021 03:30

Business, 18.03.2021 03:30


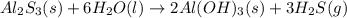
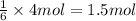 of aluminum sulfide
of aluminum sulfide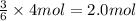 of hydrogen sulfide
of hydrogen sulfide

