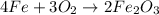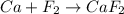Chemistry, 29.08.2019 04:30 thompsonhomes1
Classify the following reactions as either redox, acid-base, or precipitation. a) (cl2) + (2oh-) --> (cl-) + (clo-) + (h2o). b) already got. c) (nh3) + (h+) --> (nh4+). d) (4fe) + (3o2) --> (2fe2o3). e) (ca) + (f2) --> (caf2)

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 15:00
__ _ _ _ _ is the process of removing earth materials from their original sites through weathering and transport and depositing the in another location. a. erosion b. sedimentation c. lithification d. dissolution
Answers: 1

Chemistry, 22.06.2019 00:30
This active feature of earth's crust in building mountain ranges as well as islands. this feature is a a) cavern. b) earthquake. c) mountain. d) volcano.
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

You know the right answer?
Classify the following reactions as either redox, acid-base, or precipitation. a) (cl2) + (2oh-) --&...
Questions


Mathematics, 05.05.2020 05:21



Mathematics, 05.05.2020 05:21

Physics, 05.05.2020 05:21


Health, 05.05.2020 05:21


Biology, 05.05.2020 05:21


Mathematics, 05.05.2020 05:21

Mathematics, 05.05.2020 05:21


Physics, 05.05.2020 05:21




Mathematics, 05.05.2020 05:21

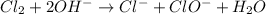
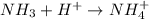
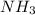 is a base and
is a base and 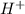 is an acid react to give
is an acid react to give 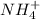 as a salt.
as a salt.