Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3

Chemistry, 22.06.2019 09:20
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3


Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
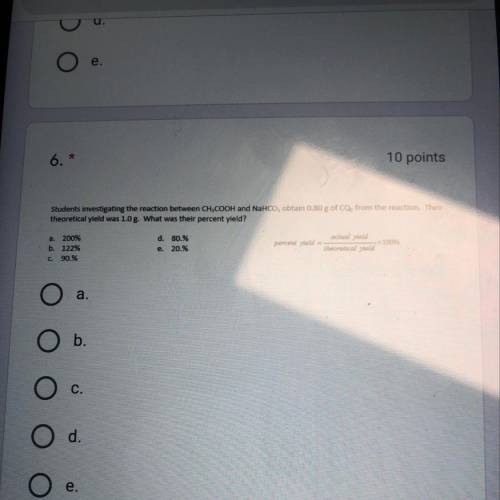
Students investigating the reaction between CH3COOH and NaHCO3 obtain .80 g of CO2 from the reaction...
Questions


Mathematics, 26.10.2020 18:30




Arts, 26.10.2020 18:30


Mathematics, 26.10.2020 18:30

Social Studies, 26.10.2020 18:30


History, 26.10.2020 18:30

History, 26.10.2020 18:30


Mathematics, 26.10.2020 18:30


Mathematics, 26.10.2020 18:30


Social Studies, 26.10.2020 18:30

Social Studies, 26.10.2020 18:30