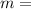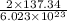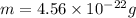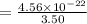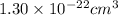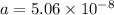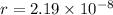Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 04:00
What three natural resources are found in the great lakes region
Answers: 2

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 09:40
Consider this initial-rate data at a certain temperature for the reaction described by
Answers: 1
You know the right answer?
The atoms in barium metal are arranged in a bodycentered cubic unit cell. Calculate the radius of a...
Questions


Mathematics, 11.10.2020 02:01





History, 11.10.2020 02:01


Chemistry, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01


English, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01



Mathematics, 11.10.2020 02:01

Mathematics, 11.10.2020 02:01


Computers and Technology, 11.10.2020 02:01

 10^-8
10^-8