
Chemistry, 02.03.2020 22:59 hoopstarw4438
At a certain temperature, 3.67 mol SO 2 and 1.83 mol O 2 are placed in a container. 2 SO 2 ( g ) + O 2 ( g ) − ⇀ ↽ − 2 SO 3 ( g ) At equilibrium, there is 1.92 mol SO 3 present. Determine the number of moles of SO 2 and O 2 that are present when the reaction is at equilibrium.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3

Chemistry, 22.06.2019 04:40
Silver tarnishes as silver metal reacts with hydrogen sulfide, h2s, in the air. in this reaction, dark silver sulfide, au2s, covers the surface of silver. when silver is polished, this coating of silver sulfide can be removed from the surface. this makes the silver shiny again. enter the coefficients that balance the tarnishing reaction equation. (type 1 for no coefficient.)
Answers: 2


Chemistry, 22.06.2019 07:00
The organism shown is a free-living one that is anchored to the bottom of ponds and streams during one stage of its life cycle what is the common name for the group to which this organism belong
Answers: 3
You know the right answer?
At a certain temperature, 3.67 mol SO 2 and 1.83 mol O 2 are placed in a container. 2 SO 2 ( g ) + O...
Questions

Mathematics, 06.05.2020 17:10



Biology, 06.05.2020 17:10

Biology, 06.05.2020 17:10

Mathematics, 06.05.2020 17:10


Mathematics, 06.05.2020 17:10

Physics, 06.05.2020 17:10





Mathematics, 06.05.2020 17:10


History, 06.05.2020 17:10


English, 06.05.2020 17:10

English, 06.05.2020 17:10

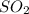 and
and 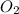 at equilibrium is, 1.75 mol and 0.87 mol respectively.
at equilibrium is, 1.75 mol and 0.87 mol respectively.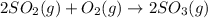
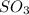 at equilibrium = 1.92
at equilibrium = 1.92

