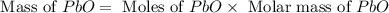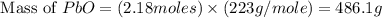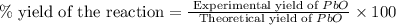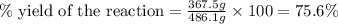Chemistry, 02.03.2020 16:32 StupidFatChipmunk
Consider the reaction. 2 Pb ( s ) + O 2 ( g ) ⟶ 2 PbO ( s ) An excess of oxygen reacts with 451.4 g of lead, forming 367.5 g of lead(II) oxide. Calculate the percent yield of the reaction.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 18:00
Mercury turns to vapor at 629.88 k how much heat is lost 175 g of mercury vapor at 650 current condenses to a liquid at 297 ca mercury turns to weber at 629.88 kelvin how much he is lost 175 g of mercury vapor and 650 coming condensers to liquidate 297 kevin
Answers: 2


Chemistry, 22.06.2019 22:00
If a solution contains 3 moles/liter of sodium chloride (nacl, made of sodium ions and chloride ions), what is the osmolarity of this solution
Answers: 3

Chemistry, 22.06.2019 23:00
Consider the reaction: 2al(s) + fe2o3(s) → al2o3(s) + 2fe(s) the δhf for fe2o3(s) = -824.3 kj/mole. the δhf for al2o3(s) = -1675.7 kj/mole. finish the equation. δhrxn = [(1)( kj/mole) + (2)( kj/mole)] - [(1)( kj/mole) + (2) ( kj/mole)]
Answers: 1
You know the right answer?
Consider the reaction. 2 Pb ( s ) + O 2 ( g ) ⟶ 2 PbO ( s ) An excess of oxygen reacts with 451.4 g...
Questions

English, 03.11.2019 18:31

Mathematics, 03.11.2019 18:31


Mathematics, 03.11.2019 18:31

Mathematics, 03.11.2019 18:31


Mathematics, 03.11.2019 18:31



Physics, 03.11.2019 18:31

English, 03.11.2019 18:31


History, 03.11.2019 18:31

Physics, 03.11.2019 18:31


English, 03.11.2019 18:31


Computers and Technology, 03.11.2019 18:31


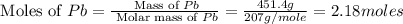
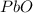
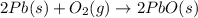
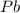 react to give 2 mole of
react to give 2 mole of