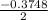Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
If you chip a tooth, most likely you will go to the dentist to have the missing material filled in. currently the material used to fill in teeth is a polymer that is flexible when put in, yet is hardened to the strength of a tooth after irradiation with blue light at a wavelength of 461 nm. what is the energy in joules for a photon of light at this wavelength?
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1

Chemistry, 23.06.2019 01:00
Which of the following is a physical change? a.burning a piece of wood b.sawing a piece of wood in half c.rust forming on an iron fence d.a copper roof changing color from orange to green
Answers: 1
You know the right answer?
Calculate the number of moles of Cl2 produced at equilibrium when 3.98 mol of PCl5 is heated at 283....
Questions

English, 27.08.2019 13:30



English, 27.08.2019 13:30

Mathematics, 27.08.2019 13:30

History, 27.08.2019 13:30






Social Studies, 27.08.2019 13:30

Mathematics, 27.08.2019 13:30




Mathematics, 27.08.2019 13:30

History, 27.08.2019 13:30

Health, 27.08.2019 13:30

Mathematics, 27.08.2019 13:30

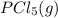 ⇄
⇄ 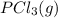 +
+ 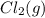
![\frac{[PCl_3][Cl_2]}{[PCl_5]}](/tpl/images/0529/9897/247b7.png)
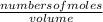
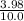
![\frac{[x][x]}{[0.398-x]}](/tpl/images/0529/9897/2afb6.png)
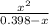
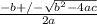
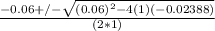
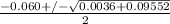
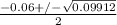
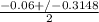
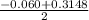 or
or 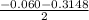
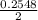 or
or