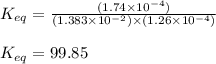Chemistry, 29.02.2020 02:52 eriksprincess13
An aqueous solution was prepared at 21 oC by mixing 7.00 mL 2.00 x 10-2mol L-1Fe3+, 2.00 mL 1.50 x 10-3 mol L-1SCN−, and 1.00 mL water. At equilibrium, the concentration of the product complex, [Fe(SCN)2+]eq was determined to be 1.74 x 10-4mol L-1. What is the value of the equilibrium constant K for the reaction of interest at 21 oC?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1



Chemistry, 22.06.2019 15:30
What best discribes the relationship between wavelength and frequency in a electromagnetic wave
Answers: 1
You know the right answer?
An aqueous solution was prepared at 21 oC by mixing 7.00 mL 2.00 x 10-2mol L-1Fe3+, 2.00 mL 1.50 x 1...
Questions

Business, 30.11.2020 18:50

Biology, 30.11.2020 18:50


Mathematics, 30.11.2020 18:50





English, 30.11.2020 18:50



English, 30.11.2020 18:50

Social Studies, 30.11.2020 18:50







Engineering, 30.11.2020 18:50

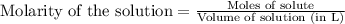 .....(1)
.....(1)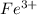 ions:
ions: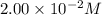
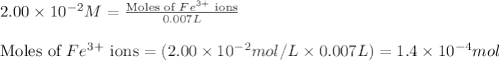
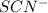 ions:
ions: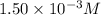
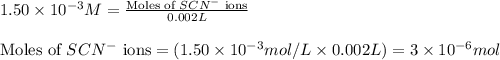
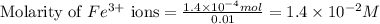
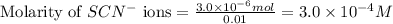
![[FeSCN^{2+}]](/tpl/images/0528/9422/797d4.png) complex follows:
complex follows:![Fe^{2+}+SCN^-\rightleftharpoons [FeSCN^{2+}]](/tpl/images/0528/9422/8a467.png)
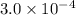
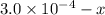 x
x![[FeSCN^{2+}]=1.74\times 10^{-4}M=x](/tpl/images/0528/9422/2c1de.png)
![[Fe^{2+}]\text{ ions}=(1.4\times 10^{-2}-x)=(1.4-0.0174)\times 10^{-3}=1.383\times 10^{-2}M](/tpl/images/0528/9422/f1fb2.png)
![[SCN^{-}]\text{ ions}=(3.0\times 10^{-4}-x)=(3.0-1.74)\times 10^{-4}=1.26\times 10^{-4}M](/tpl/images/0528/9422/6910c.png)
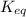 for above equation follows:
for above equation follows:![K_{eq}=\frac{[FeSCN^{2+}]}{[Fe^{3+}][SCN^-]}](/tpl/images/0528/9422/63e7c.png)