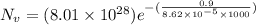Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 12:00
There is one girl i like and i don't know how to tell her that, i have a feeling she knows but if she doesn't i don't want to make a fool out of myself how is one way to boost my confidence on asking her out
Answers: 1

Chemistry, 22.06.2019 17:10
In which block of the periodic table is uranium (u) found? s blockd blockp blockf block
Answers: 1

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1

Chemistry, 23.06.2019 03:00
Asample of sea water contains 6.28g of sodium chloride per litre of solution. how many milligrams of sodium chloride would be contained in 15.0ml of this solution?
Answers: 3
You know the right answer?
Calculate the equilibrium number of vacancies per cubic meter for copper at 1000K. The energy for va...
Questions




Mathematics, 14.04.2021 22:20

Mathematics, 14.04.2021 22:20




Mathematics, 14.04.2021 22:20


Mathematics, 14.04.2021 22:20




Mathematics, 14.04.2021 22:20

Mathematics, 14.04.2021 22:20

English, 14.04.2021 22:20



Mathematics, 14.04.2021 22:20

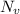 .
.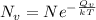
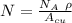
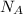 = Avogadro Number = 6.023×10²³
= Avogadro Number = 6.023×10²³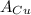 = 63.5 g/mole
= 63.5 g/mole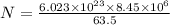
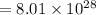 g/mole
g/mole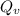 =0.9 ev/atom , T= 1000k
=0.9 ev/atom , T= 1000k