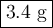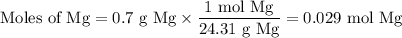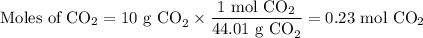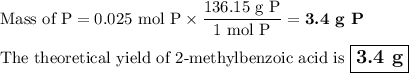Chemistry, 29.02.2020 00:11 reddmeans6
If you react 0.025 moles of 2-methylbromobenzene, 0.7 grams of Mg, and 10 grams of dry ice, what is the theoretical yield of 2-methylbenzoic acid in grams?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
How many liters of hydrogen gas will be produced at stp from the reaction of 7.179×10^23 atoms of magnesium with 54.219g of phosphoric acid (h3po4) the equation is 3mg + 2h3(> mg(po4)2+3h2
Answers: 1

Chemistry, 22.06.2019 10:30
Acompound has a molar mass of 92.02 grams/mole, and its percent composition is 30.4% nitrogen (n) and 69.6% oxygen (o). what is its molecular formula? a. n2o4 b. no2 c. n2o d. n4o2
Answers: 1

Chemistry, 22.06.2019 10:40
Asolid that forms and separates from a liquid mixture is called
Answers: 2

Chemistry, 22.06.2019 19:20
Consider hydrogen in an excited state n = 5n=5 that emits photons to reach the ground state. there are various possible transitions other than straight to the ground state that can occur; for example, it can drop to the n = 3n=3 state followed by the n = 3n=3 to the ground state transition. which of the possible transitions will result in the emission of a photon in the visible region?
Answers: 3
You know the right answer?
If you react 0.025 moles of 2-methylbromobenzene, 0.7 grams of Mg, and 10 grams of dry ice, what is...
Questions



Mathematics, 14.12.2020 19:40





Advanced Placement (AP), 14.12.2020 19:40


Mathematics, 14.12.2020 19:40



Mathematics, 14.12.2020 19:40


Biology, 14.12.2020 19:40

Mathematics, 14.12.2020 19:40

English, 14.12.2020 19:40


Mathematics, 14.12.2020 19:40