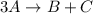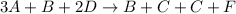Chemistry, 28.02.2020 02:26 cjjjjjjjjjjjjj
Analyzing a new reaction
Consider the following elementary steps that make up the mechanism of a certain reaction:
3A→B+C
B+2D→C+F
1. What is the overall reaction?
2. What is the rate law for step one of this reaction?
3. What is the rate law for step two of this reaction?

Answers: 3


Another question on Chemistry



Chemistry, 22.06.2019 11:00
What is the temperature of 0.750 mol of a gas stored in a 6,850 ml cylinder at 2.21 atm? . 2.95 k 5.24 k 138 k 246 k
Answers: 3

You know the right answer?
Analyzing a new reaction
Consider the following elementary steps that make up the mechanism of...
Consider the following elementary steps that make up the mechanism of...
Questions


Engineering, 22.10.2020 04:01

English, 22.10.2020 04:01


Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01

Computers and Technology, 22.10.2020 04:01

History, 22.10.2020 04:01

English, 22.10.2020 04:01

Law, 22.10.2020 04:01

Business, 22.10.2020 04:01


Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01

Mathematics, 22.10.2020 04:01



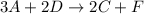
![\text{Rate}=k[A]^3](/tpl/images/0527/6240/c9f9f.png)
![\text{Rate}=k[B][D]^2](/tpl/images/0527/6240/1d2cc.png)
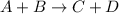
![\text{Rate}=k[A]^a[B]^b](/tpl/images/0527/6240/10aeb.png)
![[A]](/tpl/images/0527/6240/6aa06.png) and
and ![[B]](/tpl/images/0527/6240/db909.png) = concentration of A and B reactant
= concentration of A and B reactant