
Chemistry, 27.02.2020 05:24 arianaguerin
What mass of NH3 (in grams) must be used to produce 5.65 tons of HNO3 by the Ostwald process, assuming an 80.0 percent yield in each step (1 ton = 2000 lb; 1 lb = 453.6 g)? Enter your answer in scientific notation.

Answers: 1


Another question on Chemistry



Chemistry, 22.06.2019 21:00
In the experiment you asked to react hydrochloric acid and with sodium hydroxide. when measuring the volume of the reactants, which instrument would give the greatest precision.
Answers: 3

Chemistry, 22.06.2019 22:10
Determine the ph of 0.10 m nh3 solution. nh3 is a weak base with a kb equal to 1.8 x 10-5 round answer to nearest whole number.
Answers: 1
You know the right answer?
What mass of NH3 (in grams) must be used to produce 5.65 tons of HNO3 by the Ostwald process, assumi...
Questions



Biology, 04.12.2020 03:00



Mathematics, 04.12.2020 03:00

Mathematics, 04.12.2020 03:00

Mathematics, 04.12.2020 03:00

Mathematics, 04.12.2020 03:00

Chemistry, 04.12.2020 03:00

Mathematics, 04.12.2020 03:00

Mathematics, 04.12.2020 03:00

Biology, 04.12.2020 03:00


Computers and Technology, 04.12.2020 03:00

Biology, 04.12.2020 03:00


English, 04.12.2020 03:00


History, 04.12.2020 03:00

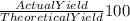 = 80 %
= 80 %

