

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:30
What is the correct term for living the most sustainable life you can within your current circumstances?
Answers: 1

Chemistry, 22.06.2019 12:00
In a laboratory, 1.55mg of an organic compound containing carbon, hydrogen, and oxygen is burned for analysis. this combustion resulted in the formation of 1.45mg of carbon dioxide and .89 mg of water. what is the empirical formula for this compound?
Answers: 1

Chemistry, 22.06.2019 13:30
Which of the following natural processes is most likely to support the formation of an underwater sinkhole? a pollution buildup from deposited minerals b limestone cave collapsing due to changes in sea level c erosion of large amounts of sand moved by ocean waves d oxidation of rock formed by chemical weathering
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1
You know the right answer?
7. A 28.4 g sample of aluminum is heated to 39.4 °C, then is placed in a calorimeter
con...
con...
Questions

History, 25.06.2019 03:30


History, 25.06.2019 03:30

Biology, 25.06.2019 03:30


English, 25.06.2019 03:30


Mathematics, 25.06.2019 03:30

Biology, 25.06.2019 03:30

English, 25.06.2019 03:30

History, 25.06.2019 03:30

Chemistry, 25.06.2019 03:30




Social Studies, 25.06.2019 03:30

Health, 25.06.2019 03:30

Physics, 25.06.2019 03:30


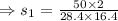
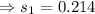 calorie /gram °C
calorie /gram °C

