
Chemistry, 27.02.2020 01:54 psychocatgirl1
Consider the reaction of ethyl acetate with sodium hydroxide: CH3COOC2H5(aq)+NaOH(aq)⇌CH3COONa(aq )+C2H5OH(aq) The reaction is first order in NaOH and second order overall. What is the rate law? View Available Hint(s) Consider the reaction of ethyl acetate with sodium hydroxide: The reaction is first order in and second order overall. What is the rate law? rate=k[CH3COOC2H5]2[NaOH]2 rate=k[CH3COOC2H5][NaOH]2 rate=k[NaOH] rate=k[CH3COOC2H5] rate=k[CH3COOC2H5][NaOH] rate=k[CH3COOC2H5]2[NaOH]

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:40
Consider the nuclear equation below. 239/94 pu—-> x+ 4/2 he. what is x?
Answers: 2

Chemistry, 22.06.2019 07:00
What effect does a decrease in temperature have on the overall rate of a chemical reaction? a decrease in temperature decreases . the reaction rate will
Answers: 1

Chemistry, 22.06.2019 12:10
If a molecule with a molecular formula of c13h18 is treated with an excess of h2 in the presence of finally divided pt metal under conditions required for maximum hydrogenation of the molecule to give a molecule with a formula c13h24, how many rings are in the molecule?
Answers: 3

You know the right answer?
Consider the reaction of ethyl acetate with sodium hydroxide: CH3COOC2H5(aq)+NaOH(aq)⇌CH3COONa(aq )+...
Questions

Biology, 03.01.2021 09:20


Mathematics, 03.01.2021 09:20



Mathematics, 03.01.2021 09:20


Biology, 03.01.2021 09:20

Physics, 03.01.2021 09:30

English, 03.01.2021 09:30

Arts, 03.01.2021 09:30


Mathematics, 03.01.2021 09:30

SAT, 03.01.2021 09:30

Mathematics, 03.01.2021 09:30





![rate=k[CH_3COOC_2H_5]^1[NaOH]^1](/tpl/images/0526/0774/1e7fe.png)
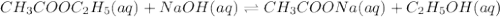
![rate=k[CH_3COOC_2H_5]^x[NaOH]^y](/tpl/images/0526/0774/4cba9.png)
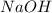
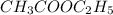 is 1 and rate law is :
is 1 and rate law is : 

