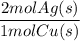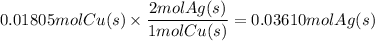Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 09:00
This chart lists four kinds of polymers and their sources. what can be known about all four polymers, despite their differences? they come from living things. they share ionic carbon bonds. they are at least 100 monomers long. they are made of repeating subunits.
Answers: 1

Chemistry, 22.06.2019 19:30
Awoman's basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). what are these specifications in units of centimeters and grams?
Answers: 2

Chemistry, 22.06.2019 22:30
How do limiting factors most affect population size? ostop population growthrestrict population growthincrease population sizeresult in positive impactso
Answers: 1

Chemistry, 23.06.2019 01:30
Which statement justifies that hydrogen peroxide (h2o2) is a polar molecule? the o – h bond is nonpolar and the molecule is asymmetric. the o – h bond is nonpolar and the molecule is symmetric. the o – h bond is polar and the molecule is asymmetric. the o – h bond is polar and the molecule is symmetric.
Answers: 1
You know the right answer?
What mass of metallic silver can form from 1.147 g of copper metal according to equation b?...
Questions


Mathematics, 08.07.2019 15:00

Mathematics, 08.07.2019 15:00



Mathematics, 08.07.2019 15:00


Chemistry, 08.07.2019 15:00

Mathematics, 08.07.2019 15:00

Mathematics, 08.07.2019 15:00

History, 08.07.2019 15:00

History, 08.07.2019 15:00


History, 08.07.2019 15:00

English, 08.07.2019 15:00

Chemistry, 08.07.2019 15:00

History, 08.07.2019 15:00

Geography, 08.07.2019 15:00


Mathematics, 08.07.2019 15:00