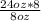Chemistry, 25.02.2020 23:17 shelbycg02
Your pharmacy stocks solution 'Q' in an 8oz concentrated bottle. Solution 'Q' is usually ordered in 8%. Consequently, your stock bottles are diluted with 16oz of distilled water to prepare 24oz of 8% solution 'Q' in a 1L glass beaker. What is the concentration of the stock bottle?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Write a paragraph at least 10 sentences explaining how rocks are used today in society
Answers: 1


Chemistry, 22.06.2019 06:10
How many moles of gas are present if p=11 atm, v=12l, t=185k?
Answers: 1

Chemistry, 22.06.2019 07:30
Given that 1 mi = 1760 yd, determine what conver- sion factor is appropriate to convert 1849 yd to miles; to convert 2.781 mi to yards.
Answers: 2
You know the right answer?
Your pharmacy stocks solution 'Q' in an 8oz concentrated bottle. Solution 'Q' is usually ordered in...
Questions

Chemistry, 28.02.2020 19:28

History, 28.02.2020 19:28



Spanish, 28.02.2020 19:28









Mathematics, 28.02.2020 19:28

English, 28.02.2020 19:28

Mathematics, 28.02.2020 19:28



History, 28.02.2020 19:28

Mathematics, 28.02.2020 19:28