
Chemistry, 28.02.2020 19:28 alexantkoviak13
The rate of disappearance of HBr in the gas phase reaction 2HBr(g)→H2(g)+Br2(g) is 0.360 Ms−1 at 150 ∘C. The rate of appearance of Br2 is Ms−1. The rate of disappearance of in the gas phase reaction is 0.360 at 150 . The rate of appearance of is . 1.39 0.600 0.720 0.180 0.130

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
How many moles are in 250 grams of tungsten (w)? * 4.4x10^23 moles 4.2x10^23 moles 0.7 moles 1.4 moles
Answers: 3

Chemistry, 22.06.2019 05:30
According to periodic trend, which of the following most likely has the highest ionization energy? kr be ni sc
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2

Chemistry, 22.06.2019 12:30
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1
You know the right answer?
The rate of disappearance of HBr in the gas phase reaction 2HBr(g)→H2(g)+Br2(g) is 0.360 Ms−1 at 150...
Questions

History, 20.10.2020 21:01

History, 20.10.2020 21:01


Social Studies, 20.10.2020 21:01



Mathematics, 20.10.2020 21:01


History, 20.10.2020 21:01


Physics, 20.10.2020 21:01

Biology, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

Physics, 20.10.2020 21:01


Mathematics, 20.10.2020 21:01

Chemistry, 20.10.2020 21:01

History, 20.10.2020 21:01

Mathematics, 20.10.2020 21:01

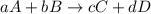
![\text{Rate of disappearance of A}=-\frac{1}{a}\frac{d[A]}{dt}](/tpl/images/0528/1964/2d8eb.png)
![\text{Rate of disappearance of B}=-\frac{1}{b}\frac{d[B]}{dt}](/tpl/images/0528/1964/1e77e.png)
![\text{Rate of formation of C}=+\frac{1}{c}\frac{d[C]}{dt}](/tpl/images/0528/1964/cee4b.png)
![\text{Rate of formation of D}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0528/1964/7ef32.png)
![Rate=-\frac{1}{a}\frac{d[A]}{dt}=-\frac{1}{b}\frac{d[B]}{dt}=+\frac{1}{c}\frac{d[C]}{dt}=+\frac{1}{d}\frac{d[D]}{dt}](/tpl/images/0528/1964/d4b94.png)
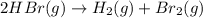
![\text{Rate of disappearance of }HBr=-\frac{1}{2}\frac{d[HBr]}{dt}](/tpl/images/0528/1964/d63dd.png)
![\text{Rate of appearance of }H_2=+\frac{d[H_2]}{dt}](/tpl/images/0528/1964/7fea8.png)
![\text{Rate of appearance of }Br_2=+\frac{d[Br_2]}{dt}](/tpl/images/0528/1964/d3d56.png)
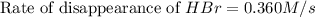
![+\frac{d[Br_2]}{dt}=-\frac{1}{2}\frac{d[HBr]}{dt}](/tpl/images/0528/1964/3e9c6.png)
![\frac{d[Br_2]}{dt}=\frac{1}{2}\times 0.360M/s](/tpl/images/0528/1964/53e73.png)
![\frac{d[Br_2]}{dt}=0.180M/s](/tpl/images/0528/1964/3fdb1.png)


