Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 06:00
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 07:00
Which set of characteristics best describes igneous rock? a) largest type of rock, made of organic matter, hardest type of rock b) least abundant type of rock, made of other rocks, made mostly of minerals c) found on all continents, contains wavy bands of stripes, contains fossils d) most abundant type in earth's crust, made of magma/lava, contains no fossils
Answers: 1

Chemistry, 22.06.2019 07:40
22. a flask containing 450 ml of 0.50 m h2so4 was accidentally knocked to the floor. how many grams of nahco, do you need to put on the spill to neutralize the acid according to the following equation: h2so4(aq)+2 nahcos(aq) na,so(aq) +2 h20()+2 co2(g) d) 38 g a) 2.3 g b) 9.5 g c) 19 g
Answers: 1

You know the right answer?
At 380 0C, the equilibrium concentrations are [CH3OH] = 0.20 M, [CO] = 0.35 M, and [H2] = 2.1 M for...
Questions

Mathematics, 16.02.2020 02:37

Mathematics, 16.02.2020 02:37



Social Studies, 16.02.2020 02:39






English, 16.02.2020 02:41

Biology, 16.02.2020 02:41

Mathematics, 16.02.2020 02:41

Mathematics, 16.02.2020 02:41

Mathematics, 16.02.2020 02:41



Mathematics, 16.02.2020 02:45


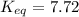
![K_{eq}=\frac{[CO]_{eq}[H_2]^2_{eq}}{[CH_3OH]_{eq}}](/tpl/images/0521/2714/cf196.png)