
Chemistry, 22.02.2020 03:33 imogengrzemskip4rq0p
. Determine the standard free energy change, ɔ(G p for the formation of S2−(aq) given that the ɔ(G p for Ag+(aq) and Ag2S(s) are 77.1 k/mole and −39.5 kJ/mole respectively, and the solubility product for Ag2S(s) is 8 10−51.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 16:30
How does decreasing the gas volume affect the pressure of a gas?
Answers: 1


Chemistry, 21.06.2019 22:30
Ibeg i need 20. a reaction produces 4.93 l of oxygen, but was supposed to produce 1 mol of oxygen. what is the percent yield?
Answers: 1

Chemistry, 23.06.2019 01:30
Which conclusion fits the data in the table? a. heat chemically changes chocolate and margarine. b. all solids become liquid at 100°f. c. removing heat from a substance it to melt. d. matter may change shape when it is heated.
Answers: 1
You know the right answer?
. Determine the standard free energy change, ɔ(G p for the formation of S2−(aq) given that the ɔ(G p...
Questions

History, 19.07.2019 20:00

Health, 19.07.2019 20:00

History, 19.07.2019 20:00

Biology, 19.07.2019 20:00



Business, 19.07.2019 20:00

Mathematics, 19.07.2019 20:00


Business, 19.07.2019 20:00

Social Studies, 19.07.2019 20:00


Health, 19.07.2019 20:00


Health, 19.07.2019 20:00





Biology, 19.07.2019 20:00

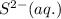 is 92.094 kJ/mol
is 92.094 kJ/mol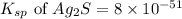
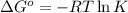
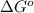 = standard Gibbs free energy = ?
= standard Gibbs free energy = ?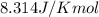
![25^oC=[273+25]K=298K](/tpl/images/0520/0871/0e82f.png)
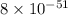
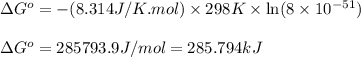
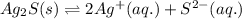
![\Delta G^o_{rxn}=\sum [n\times \Delta G^o_f_{(product)}]-\sum [n\times \Delta G^o_f_{(reactant)}]](/tpl/images/0520/0871/f2395.png)
![\Delta G^o_{rxn}=[(2\times \Delta G^o_f_{(Ag^+(aq.))})+(1\times \Delta G^o_f_{(S^{2-}(aq.))})]-[(1\times \Delta G^o_f_{(Ag_2S(s))})]](/tpl/images/0520/0871/d2b0a.png)
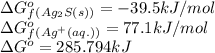
![285.794=[(2\times 77.1)+(1\times \Delta G^o_f_{(S^{2-}(aq.))})]-[(1\times (-39.5))]\\\\\Delta G^o_f_{(S^{2-}(aq.))=92.094J/mol](/tpl/images/0520/0871/afd79.png)


