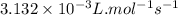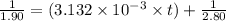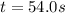Chemistry, 20.02.2020 09:40 oofoofoof1
The decomposition of NO2(g) occurs by the following bimolecular elementary reaction. 2NO2(g) → 2NO(g) + O2(g) The rate constant at 273 K is 2.3 x 10-12 L mol-1 s-1, and the activation energy is 111 kJ/mol. How long will it take (in s) for the concentration of NO2(g) to decrease from an initial partial pressure of 2.80 atm to 1.90 atm at 479 K? Assume ideal gas behavior.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:10
How many miles of water are produced if 5.43 mol pbo2 are consumed
Answers: 1

Chemistry, 22.06.2019 08:00
What is the molarity of 60.0 grams of naoh dissolved in 750 milliliters of water? a) 1.1 m b) 2.0 m c) 12 m d) 75 m
Answers: 1

Chemistry, 22.06.2019 14:00
How many absorptions would you expect to observe in the 13c nmr spectra of the following molecules? a) 3-chloropentane b) cis-4-methyl-2-pentene
Answers: 2

Chemistry, 23.06.2019 03:20
What kind of intermolecular forces act between a hydrogen fluoride molecule and a hydrogen peroxide molecule? note: if there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force.
Answers: 1
You know the right answer?
The decomposition of NO2(g) occurs by the following bimolecular elementary reaction. 2NO2(g) → 2NO(g...
Questions

Mathematics, 06.10.2019 09:01



Business, 06.10.2019 09:01


Mathematics, 06.10.2019 09:01


Mathematics, 06.10.2019 09:01




History, 06.10.2019 09:01



Mathematics, 06.10.2019 09:01

Mathematics, 06.10.2019 09:01

History, 06.10.2019 09:01

Mathematics, 06.10.2019 09:01


![\ln(\frac{K_{479K}}{K_{273K}})=\frac{E_a}{R}[\frac{1}{T_1}-\frac{1}{T_2}]](/tpl/images/0517/3905/b7df8.png)
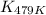 = equilibrium constant at 479 K = ?
= equilibrium constant at 479 K = ?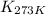 = equilibrium constant at 273 K =
= equilibrium constant at 273 K = 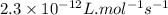
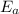 = Activation energy = 111 kJ/mol = 111000 J/mol (Conversion factor: 1 kJ = 1000 J)
= Activation energy = 111 kJ/mol = 111000 J/mol (Conversion factor: 1 kJ = 1000 J)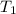 = initial temperature = 273 K
= initial temperature = 273 K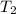 = final temperature = 479 K
= final temperature = 479 K![\ln(\frac{K_{479K}}{2.3\times 10^{-12}})=\frac{111000J}{8.314J/mol.K}[\frac{1}{273}-\frac{1}{479}]\\\\K_{479K}=3.132\times 10^{-3}L.mol^{-1}s^{-1}](/tpl/images/0517/3905/9840b.png)
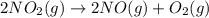
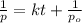
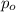 = initial partial pressure = 2.80 atm
= initial partial pressure = 2.80 atm