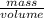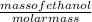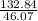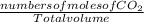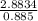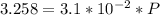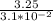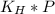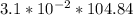Chemistry, 19.02.2020 01:04 hilario4785
Fermentation of 826 mL grape juice (density is 1.0 ) is allowed to take place in a bottle with a total volume of 885 mL until 19% by volume is ethanol (). Assuming that obeys Henry’s law, calculate the partial pressure of in the gas phase and the solubility of in the wine at 25°C. The Henry’s law constant for is mol/L ⋅ atm at 25°C with Henry’s law in the form , where is the concentration of the gas in mol/L. (The density of ethanol is 0.79 .)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 18:40
Determine the mass of fuel required for the expected energy consumption in the united states for the next ten years. energy use per person per year in the united states = 3.5 x 1011joules base calculations on current population of 310,000,000.
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2

Chemistry, 22.06.2019 11:30
Determine the reaction and balance the following equations urgent due in the morning
Answers: 2
You know the right answer?
Fermentation of 826 mL grape juice (density is 1.0 ) is allowed to take place in a bottle with a tot...
Questions

English, 27.10.2021 19:30


Mathematics, 27.10.2021 19:30

English, 27.10.2021 19:30

English, 27.10.2021 19:30





History, 27.10.2021 19:30

Mathematics, 27.10.2021 19:30



Mathematics, 27.10.2021 19:30