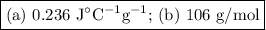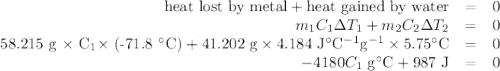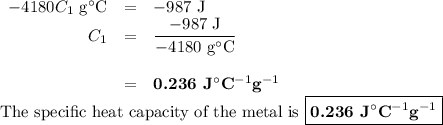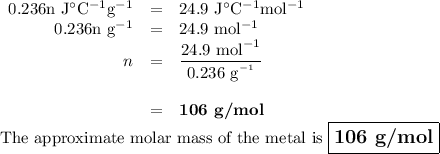Chemistry, 19.02.2020 01:04 larenhemmings
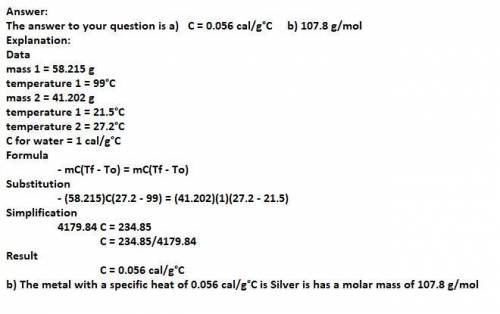
A 58.215 g sample of a pure metal is brought to 99.0c and added o 41.202 g of water at 21.5c in a calorimeter. if the metal and water arrive at a final, equal temerature of 27.2c find a) the specific heat of the metal, and b) the approximate molar mass of yhe metal

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:50
Choose all that apply. when creating a graph, you should: determine the x- and y- variables label the scale on the x- and y- axes plot the data points draw a line of best fit to represent the data trend
Answers: 1

Chemistry, 21.06.2019 22:30
Which feature do highland climates have that lower elevation areas do not?
Answers: 1

Chemistry, 22.06.2019 00:30
What does x represent in the formula for the compound xcl4?
Answers: 1

Chemistry, 22.06.2019 08:30
How does the principle of electromagnetism explain the interaction between earth’s magnetic field and the solar wind?
Answers: 1
You know the right answer?
A 58.215 g sample of a pure metal is brought to 99.0c and added o 41.202 g of water at 21.5c in a ca...
Questions

Mathematics, 24.06.2020 02:01

Mathematics, 24.06.2020 02:01

Mathematics, 24.06.2020 02:01



Mathematics, 24.06.2020 02:01


Mathematics, 24.06.2020 02:01

Biology, 24.06.2020 02:01








Computers and Technology, 24.06.2020 02:01