
Chemistry, 18.02.2020 22:31 iceecole6570
Consider the reaction 5Br−(aq)+BrO−3(aq)+6H+(aq)→3Br2(aq) +3H2O(l) The average rate of consumption of Br− is 2.06×10−4 M/s over the first two minutes. What is the average rate of formation of Br2 during the same time interval? Express your answer with th

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
What happens when the temperature of a solution increases?
Answers: 2

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 15:40
The table below shows the freezing points of four substances. substance freezing point (°c) benzene 5.5 water 0 butane –138 nitrogen –210 the substances are placed in separate containers at room temperature, and each container is gradually cooled. which of these substances will solidify before the temperature reaches 0°c? benzene water butane nitrogen
Answers: 2

Chemistry, 23.06.2019 19:10
Which of the following questions can use science as a method of inquiry? is cloning appropriate? how can we protect crops from drought? why is jazz a great type of music? did dinosaurs exist?
Answers: 2
You know the right answer?
Consider the reaction 5Br−(aq)+BrO−3(aq)+6H+(aq)→3Br2(aq) +3H2O(l) The average rate of consumption o...
Questions

Mathematics, 01.02.2021 18:20

Mathematics, 01.02.2021 18:20

Mathematics, 01.02.2021 18:20


Spanish, 01.02.2021 18:20

Mathematics, 01.02.2021 18:20

Biology, 01.02.2021 18:20

Physics, 01.02.2021 18:20



Biology, 01.02.2021 18:20


Physics, 01.02.2021 18:20

Mathematics, 01.02.2021 18:20




Mathematics, 01.02.2021 18:30

History, 01.02.2021 18:30

Mathematics, 01.02.2021 18:30

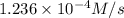 is the average rate of formation of bromine gas during the same time interval.
is the average rate of formation of bromine gas during the same time interval.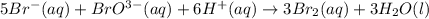
![Br^-=-\frac{d[Br^-]}{dt}= 2.06\times 10^{-4} M/s](/tpl/images/0514/7541/2309f.png)
![R=\frac{-1}{5}\frac{d[Br^-]}{dt}](/tpl/images/0514/7541/8ebea.png)
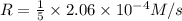

![Br_2=\frac{d[Br_2]}{dt}](/tpl/images/0514/7541/0afba.png)
![R=\frac{1}{3}\frac{d[Br_2]}{dt}](/tpl/images/0514/7541/e4812.png)
![\frac{d[Br_2]}{dt}=R\times 3=4.12\times 10^{-5}M/s\times 3=1.236\times 10^{-4} M/s](/tpl/images/0514/7541/aba02.png)


