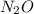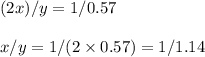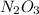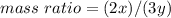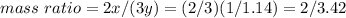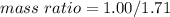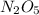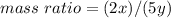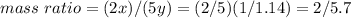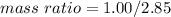Chemistry, 18.02.2020 22:23 kenndyllll
Nitrous oxide, N 2 O , has a mass ratio of nitrogen to oxygen of 1.00 : 0.57 . Determine the ratio by mass of nitrogen to oxygen in dinitrogen trioxide, N 2 O 3 , and dinitrogen pentoxide, N 2 O 5 . N 2 O 3 has a mass ratio of nitrogen to oxygen of 1.00 : N 2 O 5 has a mass ratio of nitrogen to oxygen of 1.00 :

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
This graph gives information on changes in sea ice extent in the arctic ocean over a 30-year span. the overall trend shows in the ice extent. to address the trend, scientists need to ask themselves, one direct consequence of the trend is that
Answers: 1


Chemistry, 23.06.2019 04:31
Areaction is first order. if the initial reactant concentration is 0.0200 m, and 25.0 days later the concentration is 6.25 x 10-4 m, then its half-life is:
Answers: 1

Chemistry, 23.06.2019 06:00
•what conclusions can you make about the relationship between the volume of a gas and its temperature? • what conclusions can you make about the relationship between the volume of a gas and its pressure? • what possible variables have you not accounted for? as you did the procedures, is it possible that the atmospheric pressure may have changed? if it did change over the course of your experiment, then how would your results have been affected?
Answers: 3
You know the right answer?
Nitrous oxide, N 2 O , has a mass ratio of nitrogen to oxygen of 1.00 : 0.57 . Determine the ratio b...
Questions



Social Studies, 12.11.2019 20:31











Biology, 12.11.2019 20:31