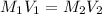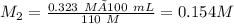Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 01:00
Which statement correctly describes potassium iodide, ki? there is a one-to-one ratio of potassium ions to iodide ions. potassium gains electrons and iodine loses electrons during the reaction. the lattice is held together by potassium anions and iodide cations.
Answers: 1


Chemistry, 22.06.2019 11:50
Calculate the molarity of each of the following solutions. part a) 0.12 mol of lino3 in 5.5 l of solution part b) 60.7 g c2h6o in 2.48 l of solution part c) 14.2 mg ki in 100 ml of solution
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1
You know the right answer?
A 100. mL solution of NaOH has a pH of 13.51. If you add 110 mL of water to the original solution, w...
Questions


Mathematics, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50


Mathematics, 20.01.2021 20:50

English, 20.01.2021 20:50


Biology, 20.01.2021 20:50


Mathematics, 20.01.2021 20:50





Mathematics, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

English, 20.01.2021 20:50

Mathematics, 20.01.2021 20:50

English, 20.01.2021 20:50

![[OH]=10^{-pOH}\\=10^{0.49}= 0.323 M](/tpl/images/0513/6468/22218.png)