Nitrogen monoxide, NO, reacts with hydrogen, H₂, according to the following equation.
W...


Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 10:30
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 23:00
In the reaction h2co3 (aq) + 3nh3 (aq) = 2 nh4+ (aq) + co3 2-, how many electrons are transferred?
Answers: 3

Chemistry, 23.06.2019 00:30
An unknown insoluble substance displaced the water shown. it's mass is indicated on the triple beam balance. mass = a. 694 b. 693.5 c. 693.0 d.693.8
Answers: 1
You know the right answer?
Questions

Mathematics, 29.09.2020 16:01

Mathematics, 29.09.2020 16:01

Biology, 29.09.2020 16:01

Mathematics, 29.09.2020 16:01


Mathematics, 29.09.2020 16:01






Mathematics, 29.09.2020 16:01







Mathematics, 29.09.2020 16:01

![Rate=k[NO]^2[H_2]](/tpl/images/0513/6470/5595c.png)
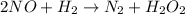 (slow)
(slow)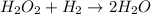 (fast)
(fast)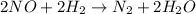
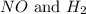 .
.


