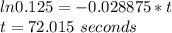The decomposition of formic acid follows first-order kinetics. HCO2H(g) → CO2(g) + H2(g) The half-life for the reaction at 550°C is 24 seconds. How many seconds does it take for the formic acid concentration to decrease by 87.5%? (1) 4.6 seconds (2) 36 seconds (3) 48 seconds (4) 72 seconds (5) 96 seconds

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 22:20
How do cfcs cause ozone depletion? how do cfcs cause ozone depletion? ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation breaks down cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks two oxygen atoms away from ozone, leaving behind a paired oxygen molecule. ultraviolet radiation creates cfcs, molecules containing chlorine. chlorine then breaks one oxygen atom away from ozone, leaving behind a paired oxygen molecule.
Answers: 2

Chemistry, 22.06.2019 22:30
Is the idea of spontaneous generation supported by redi's experiment? justify your answer in 2-3 sentences?
Answers: 1

Chemistry, 23.06.2019 00:30
In a ball-and-stick molecular model, what do the sticks represent?
Answers: 1

Chemistry, 23.06.2019 06:00
Which factor is likely to impact the possible number of compounds? presence of unlimited number of elements in the periodic table the inability of atoms to align perfectly with other atoms the ability of all elements to react with every other element all elements being equally reactive
Answers: 2
You know the right answer?
The decomposition of formic acid follows first-order kinetics. HCO2H(g) → CO2(g) + H2(g) The half-li...
Questions

Mathematics, 12.01.2020 11:31

History, 12.01.2020 11:31

Mathematics, 12.01.2020 11:31

History, 12.01.2020 11:31


Mathematics, 12.01.2020 11:31


Biology, 12.01.2020 11:31






History, 12.01.2020 11:31

English, 12.01.2020 11:31



Social Studies, 12.01.2020 11:31

Arts, 12.01.2020 11:31

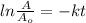
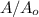 will become 0.125
will become 0.125