15 points!
I have a lab to complete and I'd like help on some yields!
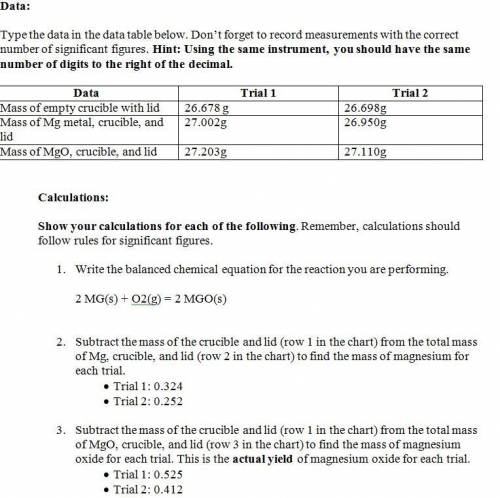
1. Mag...

15 points!
I have a lab to complete and I'd like help on some yields!
1. Magnesium is the limiting reactant in this experiment. Calculate the theoretical yield of MgO for each trial.
• Trial 1:
• Trial 2:
2. Determine the percent yield of MgO for your experiment for each trial.
• Trial 1:
• Trial 2:
Attached image: data

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Will mark brainliest26. which of these statements are true? (3 points)a. gases are compressibleb. gases fill their containers completelyc. the pressure of a gas is independent of the temperatured. gases have masse. gases exert pressuref. the pressure of a gas is dependent on the volumeg. gas pressure results from the collisions between gas particlesh. gases have a definite volume and shape
Answers: 1

Chemistry, 22.06.2019 06:30
Suppose a lab group reports a ppercent yield of sand of 105. is it really possible to collect more sand than was originally represented? what is the possible explanation for the extra product?
Answers: 2


Chemistry, 22.06.2019 10:30
What determines the average kinetic energy of the particles in a gas? a. the number of collisions b. the number of particles c. the size of the particles d. the temperature
Answers: 1
You know the right answer?
Questions

Mathematics, 04.09.2020 04:01

Mathematics, 04.09.2020 04:01


History, 04.09.2020 04:01

Physics, 04.09.2020 04:01

Mathematics, 04.09.2020 04:01


History, 04.09.2020 04:01

Mathematics, 04.09.2020 04:01

English, 04.09.2020 04:01

History, 04.09.2020 04:01




History, 04.09.2020 04:01

Mathematics, 04.09.2020 04:01



Mathematics, 04.09.2020 05:01



