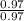Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 07:10
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 08:30
What is the independent variable in this investigation? mass volume sample number substance density
Answers: 3

Chemistry, 22.06.2019 12:40
Quiz1. which physical state of nitrogen has the highest entropy? a solid© b gasoc liquid
Answers: 1

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2
You know the right answer?
An unknown compound contains 75.69% carbon, 8.80% hydrogen, and 15.51% oxygen by mass. a mass spectr...
Questions

Mathematics, 27.01.2020 04:31


Mathematics, 27.01.2020 04:31



Mathematics, 27.01.2020 04:31

Chemistry, 27.01.2020 04:31

Mathematics, 27.01.2020 04:31



Mathematics, 27.01.2020 04:31



Computers and Technology, 27.01.2020 04:31

Mathematics, 27.01.2020 04:31

Computers and Technology, 27.01.2020 04:31


History, 27.01.2020 04:31

Biology, 27.01.2020 04:31

Mathematics, 27.01.2020 04:31

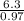 H -
H -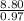 O -
O -