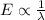Chemistry, 25.01.2020 01:31 stupidsmoke4272
In a photoelectric effect experiment, electrons are ejected from a titanium surface (work function, 3 ev) following irradiation with uv light. the energy of the incident uv light is 7.2 x 10-19 j.
(a) calculate the wavelength of the ejected electrons.
(b) calculate the wavelength of the incident uv light.
(c) would an iron surface (ф-4.7 ev require a longer or shorter wavelength of light to eject electrons with the same wavelength calculated in part (a)? briefly explain.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 21.06.2019 22:30
Write the symbol for every chemical element that has atomic number greater than 3 and atomic mass less than 12.0 u.
Answers: 1

Chemistry, 22.06.2019 03:00
About 70 percent of the earth's surface is water-covered, and about 96.5 percent of all earth's water is salt water. identify the watery feature on earth that is made of freshwater rather than salt water. a) bay b) glacier c) ocean d) sea it is not incomplete this is the true question
Answers: 1

Chemistry, 22.06.2019 10:50
Determine the empirical formula for succinic acid that is composed of 40.60% carbon, 5.18% hydrogen, and 54.22% oxygen.
Answers: 1
You know the right answer?
In a photoelectric effect experiment, electrons are ejected from a titanium surface (work function,...
Questions







History, 15.10.2019 01:10


Geography, 15.10.2019 01:10





Mathematics, 15.10.2019 01:10

Chemistry, 15.10.2019 01:10





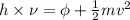
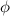 = work function
= work function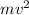 = kinetic energy of electron
= kinetic energy of electron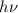 is
is 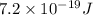 . And,
. And, 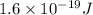
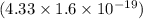 J
J  J
J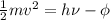
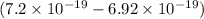 J
J J
J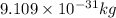 .
. 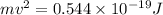
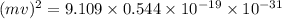


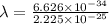
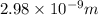
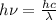
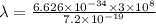
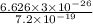
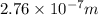
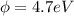
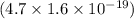 J
J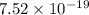 J
J