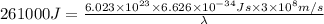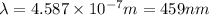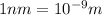Chemistry, 25.01.2020 01:31 jayowens20
It takes 261 kj/mol to eject electrons from a certain metal surface. what is the longest wavelength of light (nm) that can be used to eject electrons from the surface of this metal via the photoelectric effect?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 16:30
How many grams of mgbr2 are needed to produce 75g or metal?
Answers: 1

Chemistry, 22.06.2019 19:30
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3


Chemistry, 23.06.2019 04:30
Two liquids are poured into a beaker. after a few seconds, the beaker becomes warm. which of the following best describes this reaction? a. an exothermic reaction b. a decomposition reaction c. an endothermic reaction d. a single-displacement reaction
Answers: 1
You know the right answer?
It takes 261 kj/mol to eject electrons from a certain metal surface. what is the longest wavelength...
Questions


Biology, 08.04.2021 02:20




Mathematics, 08.04.2021 02:20


Chemistry, 08.04.2021 02:20




Mathematics, 08.04.2021 02:20

Mathematics, 08.04.2021 02:20


Mathematics, 08.04.2021 02:20



Social Studies, 08.04.2021 02:20


Mathematics, 08.04.2021 02:20

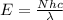
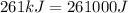 (1kJ=1000J)
(1kJ=1000J)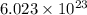
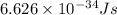
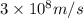
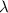 = wavelength of light = ?
= wavelength of light = ?