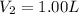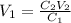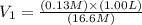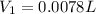Chemistry, 24.01.2020 21:31 RoyalGurl01
You wish to prepare 0.13 m hno3 from a stock solution of nitric acid that is 16.6 m. how many milliliters of the stock solution do you require to make up 1.00 l of 0.13 m hno3?

Answers: 2


Another question on Chemistry

Chemistry, 23.06.2019 01:00
Heat energy, carbon dioxide, and water are released through which process? a. photosynthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 03:10
Which of the following compounds would be expected to have the strongest ionic bonds? a)the compound that has b)the largest ions with the greatest charge c)the compound that has d)the largest ions with the least charge the compound that has the smallest ions with the greatest charge the compound that has the smallest ions with the least charge
Answers: 2

Chemistry, 23.06.2019 09:30
Which of the following is not a characteristic of a hydrogen bond? 1. it is responsible for the unusual physical properties of water. 2. it is weaker than a covalent bond. 3. it is stronger than other dipole-dipole interactions. 4. it can occur when hydrogen is covalently bound to very electronegative elements liks f, cl, br and i.
Answers: 1
You know the right answer?
You wish to prepare 0.13 m hno3 from a stock solution of nitric acid that is 16.6 m. how many millil...
Questions

Mathematics, 23.04.2020 06:20


History, 23.04.2020 06:20

Mathematics, 23.04.2020 06:20

Social Studies, 23.04.2020 06:20

History, 23.04.2020 06:20

Health, 23.04.2020 06:20

History, 23.04.2020 06:20

Mathematics, 23.04.2020 06:20

Mathematics, 23.04.2020 06:20

Computers and Technology, 23.04.2020 06:20




History, 23.04.2020 06:20

History, 23.04.2020 06:20

Mathematics, 23.04.2020 06:20



Mathematics, 23.04.2020 06:21

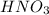
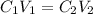
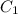 and
and 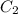 are initial and final concentration respectively.
are initial and final concentration respectively. 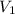 and
and 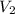 are initial and final volume respectively.
are initial and final volume respectively.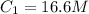 ,
, 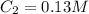 and
and