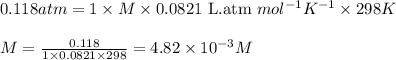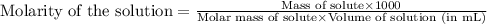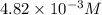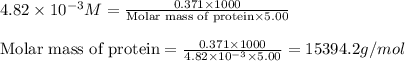Chemistry, 24.01.2020 01:31 niyyyareligion
371. mg of an unknown protein are dissolved in enough solvent to make 5.00 ml of solution. the osmotic pressure of this solution is measured to be at 0.118 atm at 25 c
calculate the molar mass of the protein.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 18:00
How is energy related to the change of state represented by the model? atoms gain energy as a solid changes to a liquid. atoms gain energy as a solid changes to a gas. atoms lose energy as a solid changes to a liquid. atoms lose energy as a solid changes to a gas.
Answers: 3

Chemistry, 22.06.2019 18:20
Which reason best explains why metals are malleable? a)because they have delocalized electrons b)because they have localized electrons c)because they have ionic bonds d)because they have rigid bonds
Answers: 2

Chemistry, 23.06.2019 01:00
Which statement characterizes synthetic polymers? a. they come from animals and plants. b. they are found in nature. c. they are made in a lab. d. they are components of starch.
Answers: 1

Chemistry, 23.06.2019 03:00
A0.100-kilogram apple hangs in a tree 1.50 meter above the ground. ignore frictional effects, the total mechanical energy of the apples is
Answers: 1
You know the right answer?
371. mg of an unknown protein are dissolved in enough solvent to make 5.00 ml of solution. the osmot...
Questions




Mathematics, 28.12.2019 10:31

Business, 28.12.2019 10:31


Mathematics, 28.12.2019 10:31

Chemistry, 28.12.2019 10:31


Physics, 28.12.2019 10:31

History, 28.12.2019 10:31




Chemistry, 28.12.2019 10:31

History, 28.12.2019 10:31



Mathematics, 28.12.2019 10:31

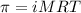
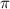 = osmotic pressure of the solution = 0.118 atm
= osmotic pressure of the solution = 0.118 atm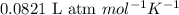
![25^oC=[273+25]=298K](/tpl/images/0468/1226/6a9f9.png)