
Chemistry, 24.01.2020 01:31 savannahvargas512
An ionic compound mx3 is prepared according to the following unbalanced chemical equation. m + x2 gives mx3, a 0.105-g sample of x2 contains 8.92 x 10^20 molecules. the compound mx3 consists of 54.47% x by mass. what are the identities of m and x, and what is the correct name for mx3? starting with 1.00 g each of m and x2, what mass of mx3 can be prepared?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 07:30
The scheme below is from a series of reactions that are part of a synthesis of vitamin a. answer the following questions with reference to this scheme. (i) what is "reagent a"? (ii) draw a step-by-step mechanism which explains the formation of compound c from compound b (iii) which reagents would you use to form compound e from compounds c and d (reagents b and c)? for each reagent suggested above in (ii) explain the role of the reagent in the reaction to (iv) form compound e. you may wish to do this by drawing a mechanism. 1. addition of reagent a но reagent a 2. н,о" thо oh нон-с compound a. compound b. compound c .ch-оh 1. reagent b "сно 2. reagent c сh oh compound e. compound d.
Answers: 2

Chemistry, 22.06.2019 13:10
The last few miles of the marathon are the most difficult for heather, her hair plastered to her head, sweat clinging to her arms, and her legs already feeling as if they had nothing left, just dead weight. after grabbing a cup of ice water, she feels the ice cubes smash against her nose as she gulps some cool refreshment and keeps on running. in these last few miles, the breeze kicks up and she finally feels some coolness against her skin. drips of sweat, once clinging to her forehead, now spill down, and heather feels more pain as the sweat flows into her eyes.which of the following is the most likely reason why the ice struck heather’s nose when she took a drink? a) water can function as a solvent. b) water can store large amounts of heat. c) water can moderate temperatures through evaporative cooling. d) the density of water decreases when it freezes. e) water has a cohesive nature.sweat remained on heather’s forehead and arms because of the a) high salt content of sweat b) cohesive nature of water c) ability of water to moderate heat d) high evaporative cooling effect of water e) ability of water to act as a solvent
Answers: 1
You know the right answer?
An ionic compound mx3 is prepared according to the following unbalanced chemical equation. m + x2 gi...
Questions


Mathematics, 07.01.2021 21:10

Mathematics, 07.01.2021 21:10

English, 07.01.2021 21:10

History, 07.01.2021 21:10


Mathematics, 07.01.2021 21:10

Mathematics, 07.01.2021 21:10

Mathematics, 07.01.2021 21:10

Mathematics, 07.01.2021 21:10



English, 07.01.2021 21:10


Mathematics, 07.01.2021 21:10



SAT, 07.01.2021 21:10

English, 07.01.2021 21:10

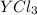 .
.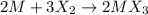
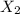 =n
=n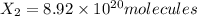
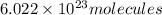
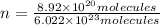
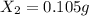
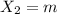
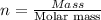
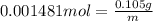
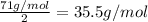
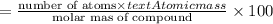
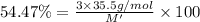
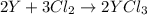
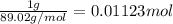
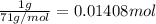
 of Y.
of Y.

